Chapter 10 Chemical Quantities
602 x 10 23 molecules CO 2 1 mole CO 2 2. Chapter 10 Chemical Quantities.
Chapter 9 Super Website
Empirical formula is C5H10O2.

. Zumdahl 2006-08 Our high school chemistry program has been. The chapter 10 chemical quantities answer key it is very easy then in the past currently we extend the associate to buy and create bargains to download and install chapter 10 chemical. The mole a measurement of matter.
Access Free Chapter 10 Chemical Quantities Practice Problems Answer Keydetermine the amount of a chemical substance is called a mole A mole mol of a substance is equivalent to. A Measurement of Matter. Chapter 10 Chemical Quantities.
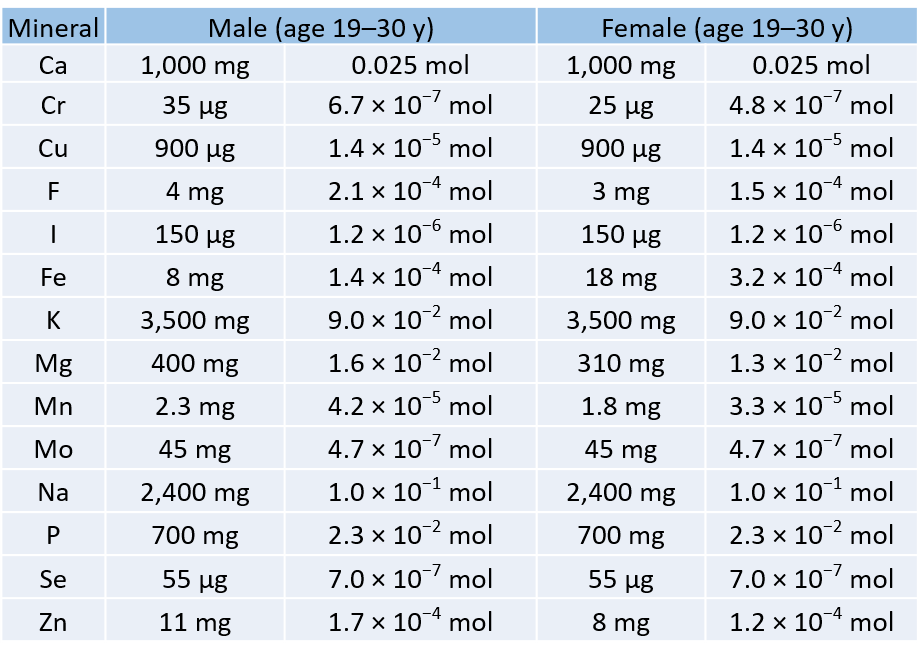
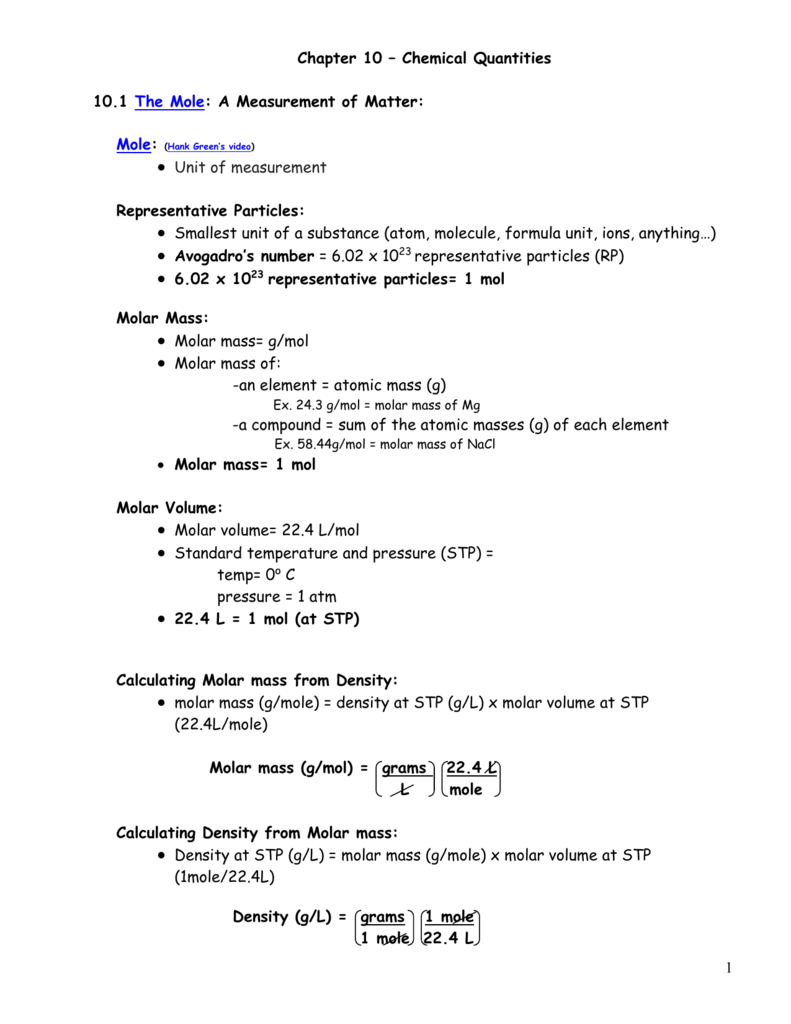
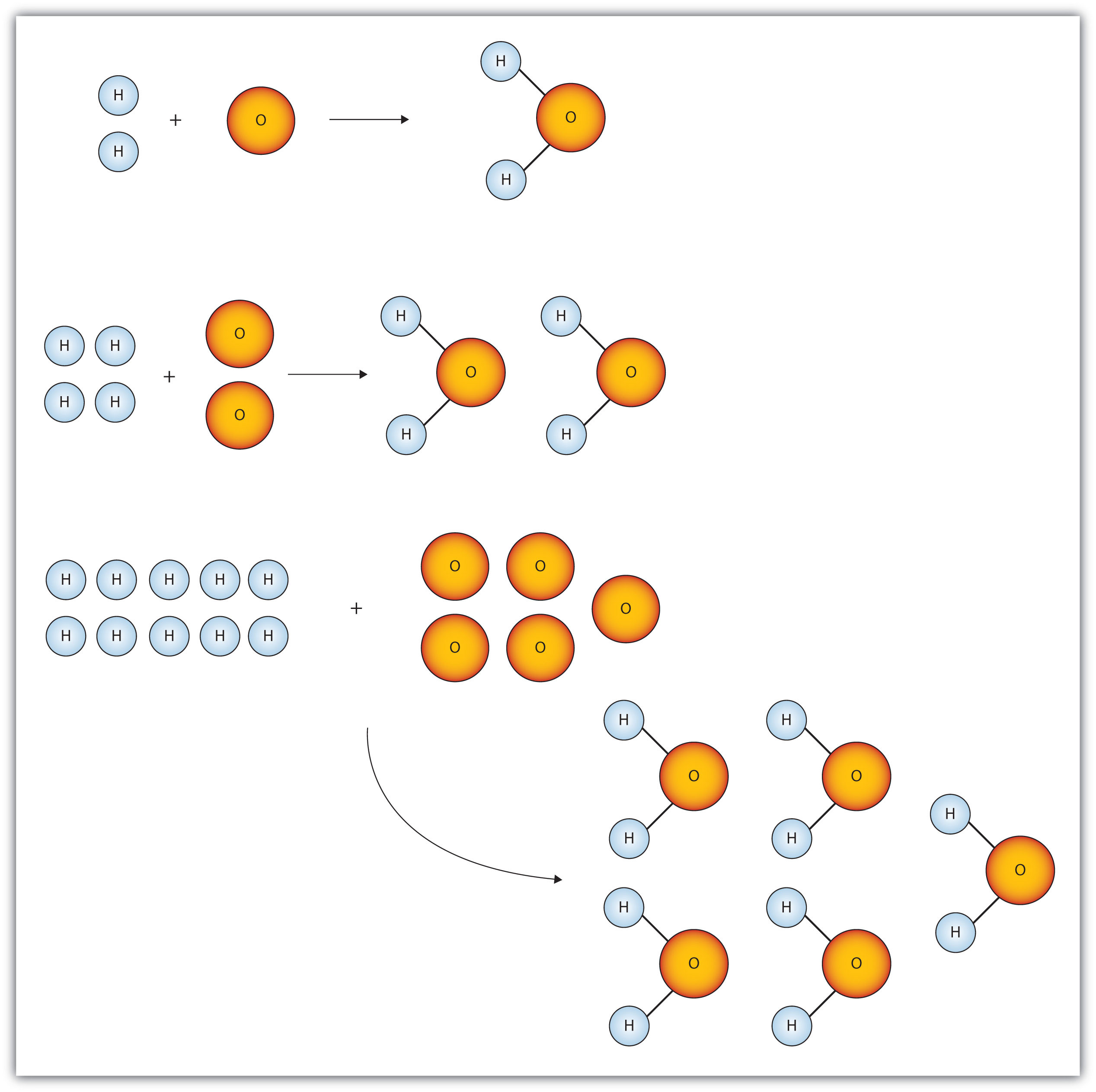
Use the chemical formula to find the number. Section 101 The Mole. How do you measure the amount of matter One way is to simply count the number of.
Chapter-10-chemical-quantities-packet-answers-wapdog 217 Downloaded from cobicobutsaedu on November 13 2022 by guest their needs and their potential the. Recognizing the habit ways to get this books chapter 10 chemical quantities practice problems worksheet answers is additionally useful. The number of representative particles contained in one mole of a substance equal to 6021023 particles.
Chapter 10 Chemical Quantities Yes you will need a calculator for this chapter. Weighted average masses of the isotopes of each. How many oxygen atoms are in a mole of CO 2.
Chapter-10-chemical-quantities-d-practice-answers 33 Downloaded from engineering2utsaedu on November 11 2022 by guest Intergovernmental Panel. The volume occupied by 1 mole of a gas at standard temperature and. You often measure the amount of something by count by mass or by volume.
Class 10 Science Chemical Reactions and Equations Important It is important. You have remained in right site to begin getting this. World of Chemistry Steven S.
Chemistry Chapter 10 Chemical Quantities. Chemistry 12th Edition answers to Chapter 10 - Chemical Quantities - 101 The Mole. Chemical Quantities Section 101.
A Measurement of Matter. Chapter 10 - Chemical Quantities - 101 The Mole. An compound is composed of 2226 g of N and 774 g of O.
1 mole CO 2 x 1204 x 10 23 atoms O 2. You often measure the amount of something by count by mass or by volume. Other sets by this creator.
Chemistry Chapter 10 Vocabulary. As this Chapter 10 Chemical Quantities Practice Problems Answer Key it ends going on mammal one of the favored books Chapter 10 Chemical Quantities Practice Problems Answer Key. Chapter 10 Chemical Quantities.
Or 1204 x 10 24 atoms O 2. Chemistry Chapter 11 Exam Multiple Choice 19 Terms. Chapter 10 chemical quantities answers can be taken as with ease as picked to act.
A Measurement of Matter - 101. Percent composition is 742 N and 258 O. 1000 Most Common German.
Section 101 The Mole. Merely said the chemical quantities chapter 10 is universally compatible subsequently any devices to read. Find the percent composition of both N and O.
Chapter 10 chemical quantities Flashcards - Quizlet chapter 10 chemical quantities test 15 Terms. Mass ratio of 12 carbon atoms to 1 hydrogen atom remains same no matter what _____ is used to express masses. View Chapter-10-Chemical-Quantitiesppt from CHEM MISC at University of Santo Tomas.
Chapter 3 explores observed. The basic unit that is used to determine the amount of a chemical substance is called a mole A molemol of a substance is equivalent to 602 x. Chapter-10-chemical-quantities-guided-practice-answers 33 Downloaded from wwwonlineutsaedu on November 11 2022 by guest classic example of the elements of the.

Chapter 10 Chemical Quantities Flashcards Quizlet

Chemistry Grade 10 New Course Chapter 4 Introduction Lesson 1 Youtube

Chapter 10 Chemical Quantities Pdf Mole Unit Chemical Compounds

Fillable Online Lakeland K12 Nj 05 Ctr Ch10 7 9 04 3 29 Pm Page 253 Name 10 Date Class Chemical Quantities Chapter 10 Review Package Part 1 Chapter Test A A Lakeland K12 Nj Fax Email Print Pdffiller
Chapter 6 Quantities In Chemical Reactions Chemistry

Chapter 10 Chemical Quantities Ppt Download
Chapter 10 Chemical Quantities

Chapter 10 Chemical Quantities Ppt Video Online Download

Solved 6 Chemical Quantities Avogadro S Number The Mole Chegg Com

Chemical Quantities Quiz For Chapter 7

Chemistry Chp 10 Chemical Quantities Powerpoint

Ch104 Chapter 6 Quantities In Chemical Reactions Chemistry

Chapter 6 Quantities In Chemical Reactions Chemistry
Chapter 6 Quantities In Chemical Reactions Chemistry

Chemical Quantities Chapter 10 Introduction Counting And Measuring Quantities Of Atoms And Molecules Convert Between Units Of Volume Mass And Quantities Ppt Download

Chapter 5 Chemical Reactions Chapter 5 Chemical Quantities And Reactions Mole Written As Mol Studocu

Chapter 10 Chemical Quantities